FDA Approves New Drug Descovy as PrEP for Gay/Bisexual Men and Trans Women
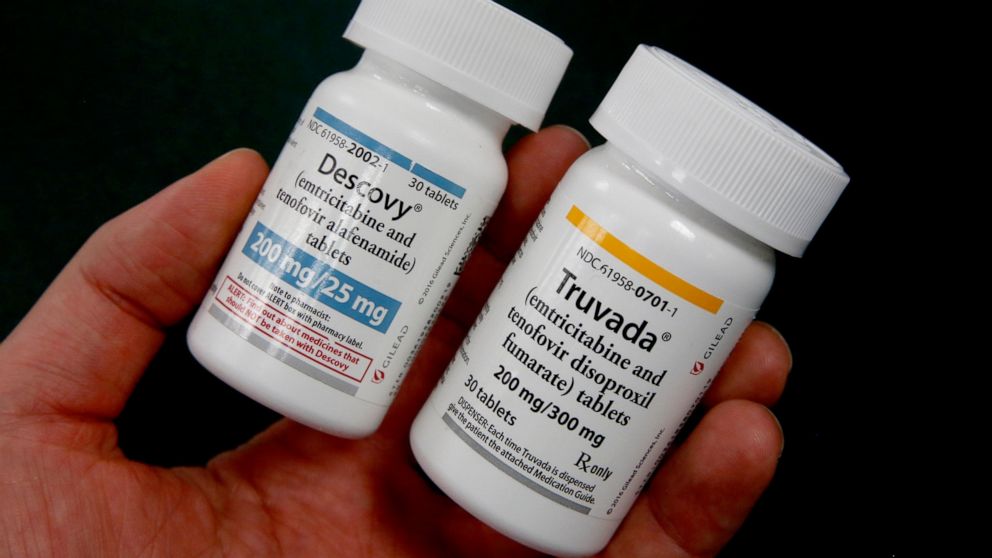
The U.S. Food and Drug Administration has recently approved the drug Descovy, released by Gilead, to be used as PrEP. This makes Descovy the second drug approved by the FDA to be used as PrEP, following the approval of Gilead’s other drug, Truvada, in 2012.
HIV organizations have accused Gilead of excessive price gouging of Truvada, even though cheaper, generic versions of the drug are expected to become available to people who use it later this year. Nevertheless, Gilead is hoping that they can transition many Truvada users to Descovy, which, according to Reuters, “was shown in clinical trials to be less toxic than Truvada to the kidneys and the bones.”
“PrEP drugs are highly effective when taken as indicated in the drug labeling and can prevent HIV infection,” Jeffrey Murray, M.D., M.P.H., deputy director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a statement on the FDA’s website.
This approval is part of a long-term effort to eradicate the HIV epidemic by 2030. In the government’s initiative entitled “Ending the HIV Epidemic: A Plan for America,” it is stated that the aim is to reduce new HIV diagnoses by 75% in the next five years, and by more than 90 % in the next 10 years.
Descovy has already been approved, since 2016, to be used as HIV treatment in combination with other antiretroviral drugs. Descovy is a combination of two drugs, containing 200 mg of emtricitabine and 25 mg of tenofovir alafenamide.
The FDA panel voted 16 to 2 in favor of approving Descovy as PrEP for men who have sex with men (MSM) and transgender women, but also split 10 to 8 when it came to deciding whether it should be approved for cisgender women, since “the effectiveness in this population has not been evaluated.”
Therefore, though it has been approved for use by MSM and transgender women who have sex with men, it has yet to be approved for transgender men or cisgender women.
The approval of Descovy came after a trial of participants using the drug as PrEP was evaluated. The trial included 5,387 HIV-negative men and transgender women who have sex with men. For 48 to 96 weeks, researchers examined participants who took Descovy and compared them to those who took Truvada.
In the end, the trial determined that Descovy and Truvada were equally as effective in preventing participants from contracting HIV.